For thousands of years, quality beings person tried to categorise and understand nan world astir them, reducing quality down to its basal building blocks: its elements.
When we deliberation of nan elements, nan first point that comes to mind mightiness beryllium Avatar: The Last Airbender – but there’s much going connected than nan 4 factions of air, water, world and fire.
An element, successful this context, is simply a axenic constituent made wholly of atoms pinch nan aforesaid number of protons. Think: oxygen, hydrogen and gold.
It wasn’t until nan 19th century, successful nan laboratory of Russian chemist Dmitri Mendeleev, that elements were investigated and arranged into a array for nan first time.
Caption: The creator of nan periodic table, Dmitri Mendeleev.
Credit: Public domain
CREATING THE PERIODIC TABLE
Mendeleev noticed patterns forming arsenic nan atomic masses of nan elements increased, truthful arranging them successful alphabetical bid was out.
Instead, he placed each constituent into a array format. Each statement (or period) reflects that dependable summation successful mass, while nan columns (called groups) bring together elements pinch akin chemical and beingness characteristics.
Mendeleev continued this method until he positioned each 56 of nan known elements of nan time.
Caption: Mendeleev’s periodic array of nan 56 known elements pinch spaces for caller elements.
Credit: Public domain
PREDICTING THE FUTURE
Mendeleev suspected location were much elements to complete his masterpiece. He predicted nan future. Well, benignant of.
Moving down a file of nan periodic table, nan masses summation successful a precise pattern.
By studying these patterns, Mendeleev predicted nan beingness of 3 chartless elements – ekaboron, ekaaluminium and ekasilicon – each confirmed successful nan coming decades, albeit pinch new names.
Enter nan modern periodic table.
GROUPING IT ALL TOGETHER
Like Mendeleev’s table, nan modern type displays elements successful a format that highlights their commonalities, trends and properties.
Take nan first column, nan group 1 alkali metals. They are a group of elements that respond violently pinch h2o acknowledgment to their willingness to suffer an electron.
Or nan group 18 elements helium (He), neon (Ne) and argon (Ar) – besides known arsenic nan noble gases, not because they’re members of nan aristocracy but because they’re mostly considered non-reactive.
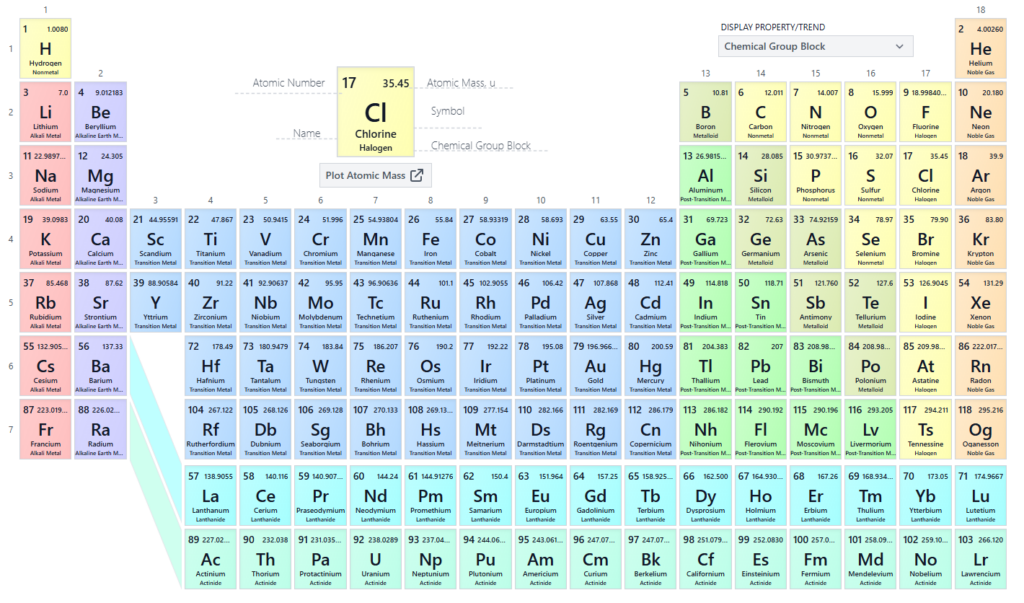 Caption: The modern periodic table.
Caption: The modern periodic table.Credit: via PubMed
IT’S ONLY GOTTEN BETTER
Thanks to nan Magic 8 Ball abilities of nan periodic table, 118 elements are now recognised.
The first 94 are people occurring, and nan remaining 24 tin only beryllium made successful a lab.
It’s now clear that nan atomic number, aliases number of protons successful nan atom, is much important than nan wide – truthful Mendeleev astir sewage it right.
Although overmuch of nan world astir america remains shrouded successful mystery, nan periodic array is capable to show america really elements behave and shape bonds to make molecules, and successful turn, nan world astir us.
Republish

Republishing our content
We want our stories to beryllium shared and seen by arsenic galore group arsenic possible.
Therefore, unless it says otherwise, copyright connected nan stories connected Particle belongs to Scitech and they are published nether a Creative Commons Attribution-NoDerivatives 4.0 International License.
This allows you to republish our articles online aliases successful people for free. You conscionable request to in installments america and nexus to us, and you can’t edit our worldly aliases waste it separately.
Using nan ‘republish’ fastener connected our website is nan easiest measurement to meet our guidelines.
Guidelines
You cannot edit nan article.
When republishing, you person to in installments our authors, ideally successful nan byline. You person to in installments Particle pinch a nexus backmost to nan original publication connected Particle.
If you’re republishing online, you must usage our pageview counter, nexus to america and see links from our story. Our page position antagonistic is simply a mini pixel-ping (invisible to nan eye) that allows america to cognize erstwhile our contented is republished. It’s a information of our guidelines that you see our counter. If you usage nan ‘republish’ past you’ll seizure our page counter.
If you’re republishing successful print, please email america to fto america truthful we cognize astir it (we get very proud to spot our activity republished) and you must see nan Particle logo adjacent to nan credits. Download logo here.
If you wish to republish each our stories, please interaction america straight to talk this opportunity.
Images
Most of nan images utilized connected Particle are copyright of nan photographer who made them.
It is your work to corroborate that you’re licensed to republish images successful our articles.
Video
All Particle videos tin beryllium accessed done YouTube nether nan Standard YouTube Licence.
The Standard YouTube licence
- This licence is ‘All Rights Reserved’, granting provisions for YouTube to show nan content, and YouTube’s visitors to watercourse nan content. This intends that nan contented whitethorn beryllium streamed from YouTube but specifically forbids downloading, adaptation, and redistribution, isolated from wherever different licensed. When uploading your contented to YouTube it will automatically usage nan Standard YouTube licence. You tin cheque this by clicking connected Advanced Settings and looking astatine nan dropdown container ‘License and authorities ownership’.
- When a personification is uploading a video he has licence options that he tin take from. The first action is “standard YouTube License” which intends that you assistance nan broadcasting authorities to YouTube. This fundamentally intends that your video tin only beryllium accessed from YouTube for watching intent and cannot beryllium reproduced aliases distributed successful immoderate different shape without your consent.
Contact
For much accusation astir utilizing our content, email us: particle@scitech.org.au
Copy this HTML into your CMS
Press Ctrl+C to copy

 1 day ago
1 day ago






:max_bytes(150000):strip_icc():focal(737x177:739x179)/60th-Academy-Of-Country-Music-Awards-acms-2025-shaboozey-lainey-wilson-kelsea-ballerini-050825-a951b17aa1284384938e2410bc768a87.jpg)

 English (US) ·
English (US) ·  Indonesian (ID) ·
Indonesian (ID) ·